Why is the Periodic Table Shaped like a Castle?
If you open any chemistry textbook, the first thing you’ll likely see is the periodic table of elements. But it doesn’t look anything like any table you’ve ever seen before. In fact it looks more like a castle than a table. So why is the periodic table shaped the way it is?
If you look at the following picture, you can see the elements are ordered left to right by the number of protons, which is indicated by the number in the upper right corner. As you go down the column, you can see that each element is ordered by the number of outer rings.
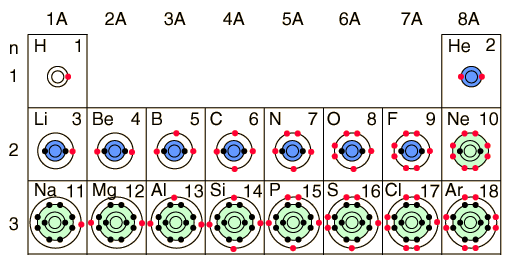 This is all fine and dandy until we inspect the periodic table closely. Although we just said elements are arranged by proton number, we notice some elements are not laid out next to one another. For example, hydrogen and helium, number 1 and number 2, are on opposite sides of the table.
This is all fine and dandy until we inspect the periodic table closely. Although we just said elements are arranged by proton number, we notice some elements are not laid out next to one another. For example, hydrogen and helium, number 1 and number 2, are on opposite sides of the table.
The spatial gaps between the elements indicate the stability of the element. Stability is determined by the number of electrons on the outer shells of the element. Electrons that have full outer shells have more in common with each other than those with partially full outer shells. The more full the outer shell is, the more stable the element. We can deduce that Helium is more stable than Hydrogen because it is further to the right.
The layout of the periodic table of elements is complex. Not only is it determined by protons, but also by each element’s chemical and physical properties.
By having the periodic table laid out in the shape of a castle, scientists can easily observe each element and its properties. If we were to order the chemical elements by alphabetical order, it would not be able to tell users anything about the properties of the elements.
